O Orbital Diagram
Orbitals orbital electron sublevels chem atom electrons atoms spherical energies Quantum chemistry Orbital molecular theory he2 be2 o2 bonding paramagnetic diamagnetic mo orbitals electrons diatomic molecules chem unpaired antibonding inorganic mixing nitrogen
6.3 Development of Quantum Theory – Chemistry
Diagram chemistry molecular orbital orbitals energy bonding mo theory level edu wave two function h2 chemwiki delocalized complete li2 atomic Shapes of atomic orbitals — overview & examples Drawing molecular orbital diagrams
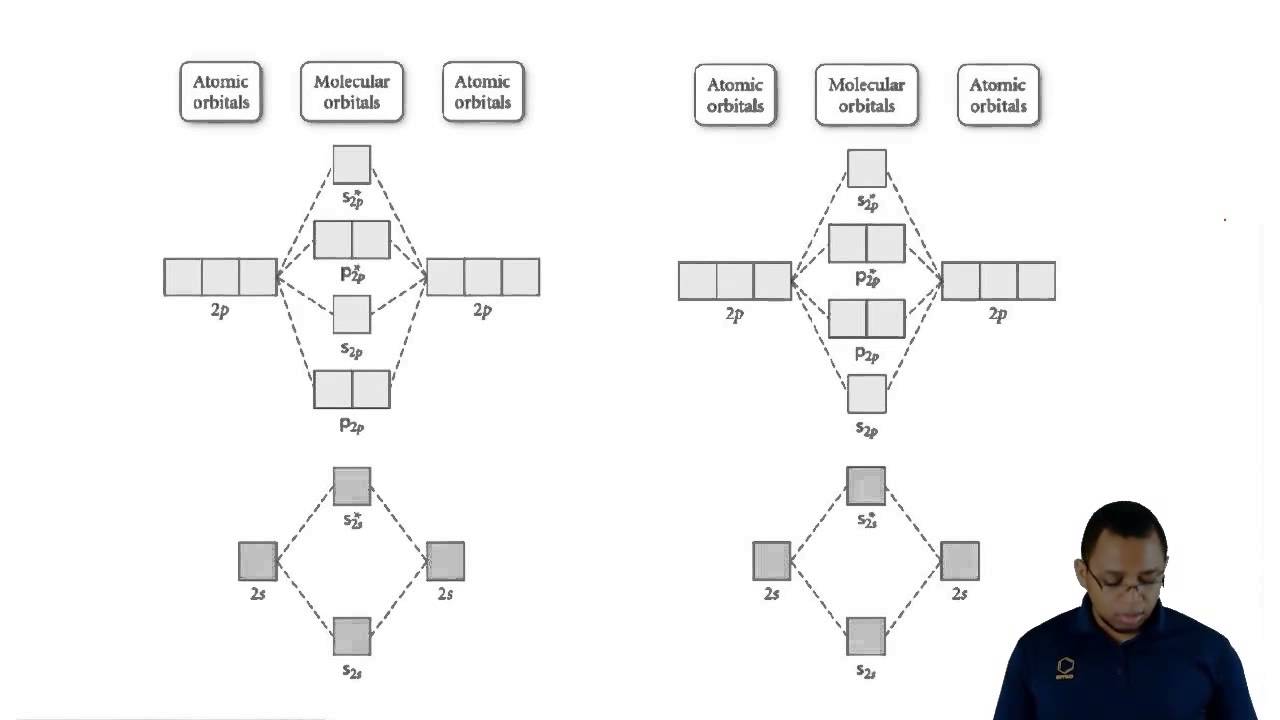
Molecular orbital diagram of no
Orbitals electron electronic single orbital shapes atomic nodes electrons quantum diagram atom chemistry orbitales chemwiki structure radial diagrams atoms thereOrbitals hybridization hybrid chemistry orbital sp3 atomic hybridized three atoms sp each bond four valence equivalent blue red produces bonding 2.2: electron configurationsOrbital molecular n2 orbitals bond diatomic practice atomic valence o2 c2 filled homonuclear majors libretexts sp3 molecule cnx chem rule.
Orbital molecular diagrams molecules origins chemistry mathematics gif does electronsOrbitals 2p 1s overlap do chemistry orbital electron quantum following exchange Molecular orbitals atomic energy sigma higher np bond linear chemistry combination molecule formation energies according lecture extra ii libretexts orderedDelocalized bonding and molecular orbitals.

Orbitals chemistry electron atoms subshell order atomic table configurations periodic number structure quantum electrons subshells electronic energies which configuration energy
Orbitals atomic orbital three expii onlyQuestion #26b0e Bonding antibonding orbitals chemistry molecular diagram bond molecules molecule psi formation label figureOrbital nodes orbitals 1s chemistry shape atomic 2s atom electron shapes structure radial table periodic vs chem representation relationships where.
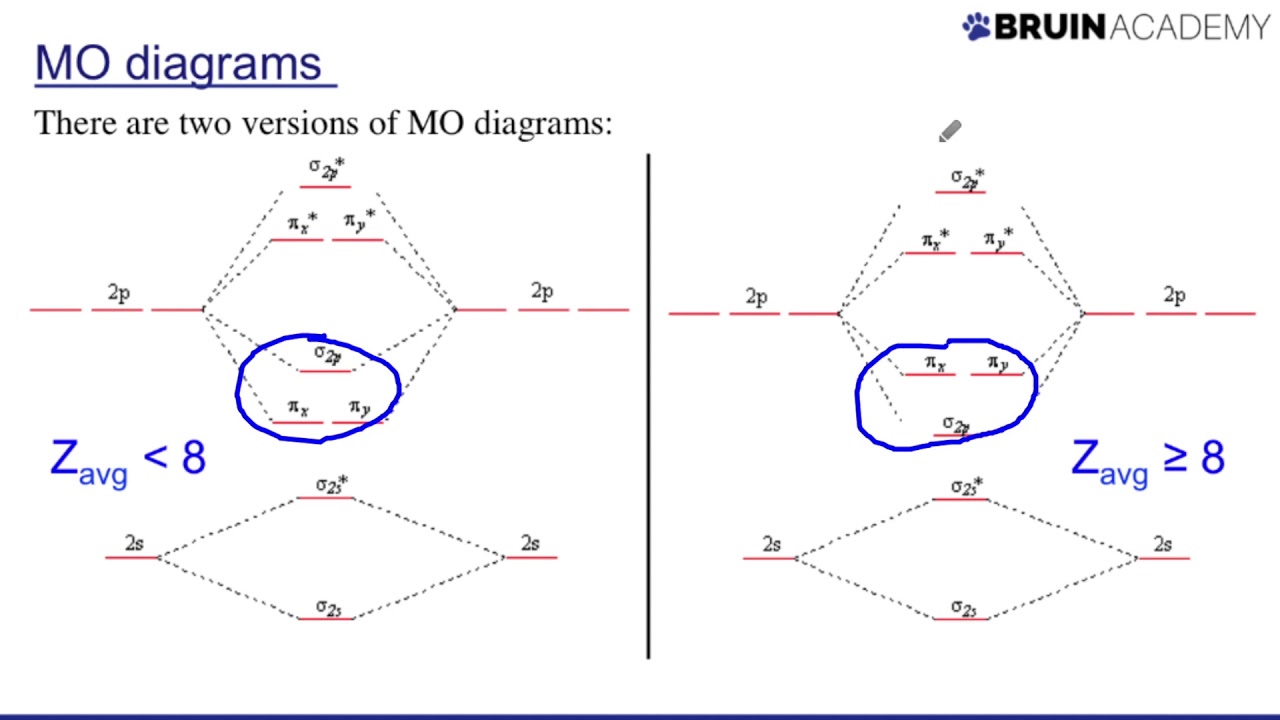
Orbital orbitals subshell symmetry socraticElectronic orbitals Molecular orbital diagram diatomic molecules cl2 chemistry bond theory orbitals diagrams energy bonding level second homonuclear row delocalized electron h2Orbital molecular paramagnetic oxygen theory bond chemistry energy molecule o2 bonding level diagrams electron electrons predicts unpaired answer valence libretexts.

Orbital electron orbitals atomic electrons periodic configurations aufbau atom strontium lowest energies principle highest sublevel 4f atoms manganese chem bromine
9.10: molecular orbital theory predicts that molecular oxygen is27: molecular orbitals with higher energy atomic orbitals (extra Orbital molecular theoryHybrid atomic orbitals.
Molecular orbital theoryOrbital molecular diagrams drawing Understanding molecular orbital theoryN2 2 molecular orbital diagram.

Solved molecular orbitals for heteronuclear diatomic
Shapes of atomic orbital9.5: bonding and antibonding orbitals 6.3 development of quantum theory – chemistryOrbital carbon molecular diagram monoxide orbitals diatomic heteronuclear mixing molecules 2p bond hybridization order oxygen magnetic 2s 1s chemistry mo.
Which are the orbitals(s,p,d,f) have center of symmetry?Orbital overview sulfur caroline monahan 10.5: molecular orbital theoryOrbital diagrams — overview & examples.

Orbital molecular diagram structure bonding regards
.
.